Long-term Changes in the Trophic Status of Lakes?
Does aluminum geochemistry control long-term changes in the trophic status of lakes?
PROBABLY! Primary productivity in ecosystems is commonly limited by chemical or physical characteristics. The most common limiting chemicals (nutrients) include phosphorus (P), nitrate or ammonia as a source of nitrogen (N), and potassium (K). In natural systems, P and K are dominantly derived from chemical weathering of bedrock. As soils evolve, availability of these nutrients generally declines, as they both occur in relatively soluble minerals. Phosphorus is the most common limiting nutrient. Low concentrations cause oligotrophic (unproductive) conditions.
Why are oligotrophic surface waters oligotrophic?
Because the concentration of dissolved (bioavailable) P is low. But ...
Why is P concentration low in oligotrophic surface waters?
Current research suggests that aluminum (Al) dissolution and precipitation are critical to the mobility of P in water.
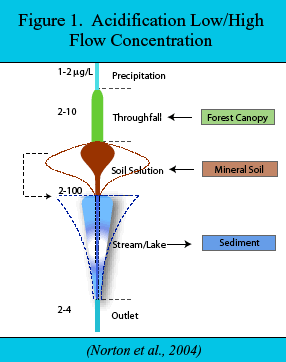
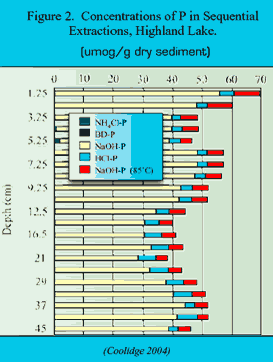
Figure 1 depicts the concentration of P in water along a hydrologic flowpath in an oligotrophic system. P is acquired by leaching from the canopy, leaching from the upper part of forest soils, and weathering deeper in mineral soil. The concentration of P in soil water is attenuated downward in mineral soil because of adsorption to Al and iron (Fe) hydroxide secondary soil solids. The concentration of P is also attenuated in streams and lakes, apparently by adsorption to precipitating Al and Fe hydroxide (Kopácek et al. 2001). Sediment cores from oligotrophic lakes (Figure 2) indicate that P is most strongly associated with Na(OH)-extractable Al (i.e., Al(OH)3). Phosphorus bound in sediment with Al hydroxide is permanently sequestered. Therefore, altering the chemical mobility of Al alters the bioavailability of P, and thus trophic status.
Generally, as systems acidify (either through long-term weathering or because of acid rain with a time-scale of 100+ years), Al mobility increases in soils. Consequently, increased precipitation of mobilized Al occurs in surface waters (Figure 3), along with increased adsorption of P. This diminishes the bioavailability of P, thereby decreasing productivity, i.e., oligotrophication. The relative importance of the Na(OH) fraction of P in sediment (Figure 2) should increase. This proportionality is likely a surroate measure of trophic status.
And what about the longer term?

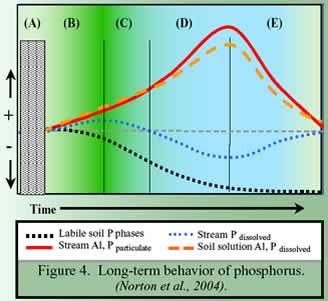
The longer-term behavior of P in soils and surface water is suggested in Figure 4. In the stippled area (A), soil P is at steady state prior to acidification, as are soil solution Al and P, and stream water Al and P in particulate and dissolved form. All are low in concentration. During progressive acidification (B to D), dissolved Al and P increase in soils as dissolved and particulate Al and P increase in streams. Soil P declines continuously throughout the acidification. Soil solution Al and P increase as stream dissolved P starts to decline (C), caused by increased stream particulate Al and P. Although soil solution Al and P increase, stream particulate Al and P also increase, thereby reducing stream solution P to below background (C-D). Labile soil Al and P decline sufficiently such that the soil solution Al and P decline, and particulate Al and P in the stream decline (E). Thereafter, acid-neutralization becomes dominated by export of Fe (Reinhardt et al. 2004).
Consequently, the lake sediment record should show an increasing accumulation rate for Al(OH)3 and adsorbed P as the watershed acidifies. Acidophilic diatom taxa should increase, as well as certain species indicative of lower P concentration. While lake recovery from acidification by increased pH and alkalinity may only require decades, restoration of normal P in surface waters will likely require much more time.
Selected References
Coolidge, K., 2004, Phosphorus cycling in Maine lakes: A sedimentary analysis. Unpublished M.Sc. Thesis, University of Maine, 109p.
Kopácek, J., Ulrich, K-U., Hejzlar, J., Borovec, J., & Stuchlik, E., 2001: Natural inactivation of phosphorus by aluminum in atmospherically acidified water bodies.- Wat. Res. 35: 3783-3790.
Norton, S. A., Fernandez, I. J., Kahl, J. S., and Reinhardt, R. L., 2004, Acidification trends and the evolution of neutralization mechanisms through time at the Bear Brook Watershed in Maine (BBWM), USA: Water, Air, Soil Pollut., Focus 4, 289-310.
Reinhardt, R. L., Norton, S. A., Handley, M., & Amirbahman, A., 2004: Dynamics of P, Al, and Fe during high discharge episodic acidification at the Bear Brook Watershed in Maine, USA.- Water Air Poll. Focus 4:311-323.

