Skip navigational links Understanding Atmospheric Chemistry
P.A. Mayewski,
Climate Change Institute and Department of Earth Sciences
Dramatic change in the chemistry of the atmosphere has occurred over the last century due to human activity.
1. From the GISP2 ice core it was determined that levels of sulfate and nitrate (the primary components of acid rain) in the North Atlantic and much of the Northern Hemisphere have greatly exceeded their natural levels as a consequence of human activity.
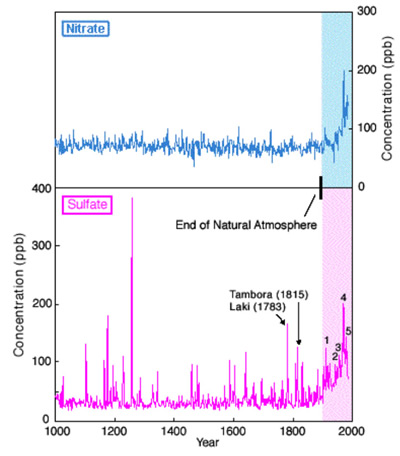
Top figure - The past 1000 years of sulfate and nitrate from the GISP2 record. Note dramatic increase in both of these major components of acid rain during the 20th century relative to levels of the past 1000 years. Changes in sulfate are closely tied to industrial activity in North America and Europe: (1) beginning of the industrial revolution, (2) the Great Depression, (3) World War II, (4) period of most intense burning of sulfur rich, "dirty" coal, and (5) beginning of the Clean Air Act. The Clean Air Act did not have a dramatic effect on nitrate levels. Most of the short- term (up to one to two years) increases in sulfate are the product of volcanic activity such as the Tambora eruption of 1815 and the Laki eruption of 1783. Data from Mayewski et al. (1986, 1990).
2. From GISP2 and South Pole it was determined that the Chernobyl nuclear accident released radioactive fallout that spread throughout the Arctic and high latitudes of the Northern Hemisphere and was even transported to the high latitude south polar region through the upper atmosphere.
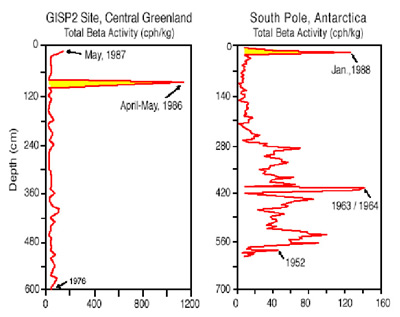
Bottom figure - Total beta radioactivity (measured in counts per hour per kilogram (cph/kg) from snow pits in central Greenland (GISP2 site) and 25 miles (40km) from the South Pole. Both snow pits were hand excavated to a depth of approximately 20 feet (6 meters) and then sampled for radioactivity. The site near the South Pole contains snow dating back to 1952 and the central Greenland snow pit only back to 1976, demonstrating the greater amount of annual snowfall in central Greenland. Snow pits containing snow that dating back to the 1950's (like the site near the South Pole) contain evidence of former atmospheric testing of nuclear bombs in the form of total beta radioactivity. These "bomb layers" provide a means for calibrating the exact age of the snow. As of the mid 1960's, atmospheric testing of nuclear bombs was banned and radioactivity levels in the atmosphere and snow dropped to natural background levels. The 1986 nuclear accident at the Chernobyl reactor in the former Soviet Union released sufficient radioactivity to contaminate much of the high latitudes of the Northern Hemisphere (see highlighted yellow levels in central Greenland snow pit), but it was not assumed that this radioactivity could extend into the Southern Hemisphere.
However, some radioactivity did rise high enough into the atmosphere to get into regions of the atmosphere where air can travel easily from high latitudes of the Northern Hemisphere to high latitudes of the Southern Hemisphere. The input timing for Chernobyl radioactive debris at South Pole is close to 19-20 months from the time of its injection into the atmosphere as indicated by the presence of high total beta radioactivity in January 1988 snow near South Pole (note highlighted yellow section in snow pit). This dreadful accident provides a marker for the time of the accident close to its source and a fingerprint for tracing the time it took this air to reach the South Pole. The transport pathway is similar to that taken by ozone-destroying chemicals that are produced by humans in the Northern Hemisphere. Although most of their production is in the Northern Hemisphere the first ozone destroying consequences occur over Antarctica.


